Science Vertical III
Science Verticals
Cross-Cutting Themes
Safety and Degradation in Sodium-Ion Chemistry
Due to the limited availability of lithium
resources and concerns related to their sustainability, sodium-ion batteries have
become an excellent candidate for applications such as stationary grid storage.
Sodium-ion batteries offer various advantages, including the high abundance,
low cost, and redox potential of sodium. Based on various aspects such as
desolvation energies, coordination preferences, solid electrolyte interphase
characteristics, and electrolyte interactions, sodium-ion chemistry can fundamentally
differ from its lithium-ion counterpart.
In addition, the role of cathode/anode
microstructure, pore-scale attributes, and electrolyte transport on the
electrochemical performance, degradation, and safety in sodium-ion batteries
will be distinct. Through this science vertical, the fundamental
kinetic-transport interactions in sodium-based electrode microstructures,
electrode-electrolyte interactions, and their implications on safety science in
sodium-ion batteries will be comprehensively studied. With respect to the
cathode, similar to lithium-ion batteries, highly reversible cathode materials
based on intercalation reactions are required to achieve high capacity and
stability in sodium-ion batteries.
Due to the larger size of sodium ions,
structural changes due to ion intercalation in the host are more prominent. The
role of such geometric deformations of electrode materials on the kinetics of
electrochemical reaction, transport, and mechanical stress distribution within
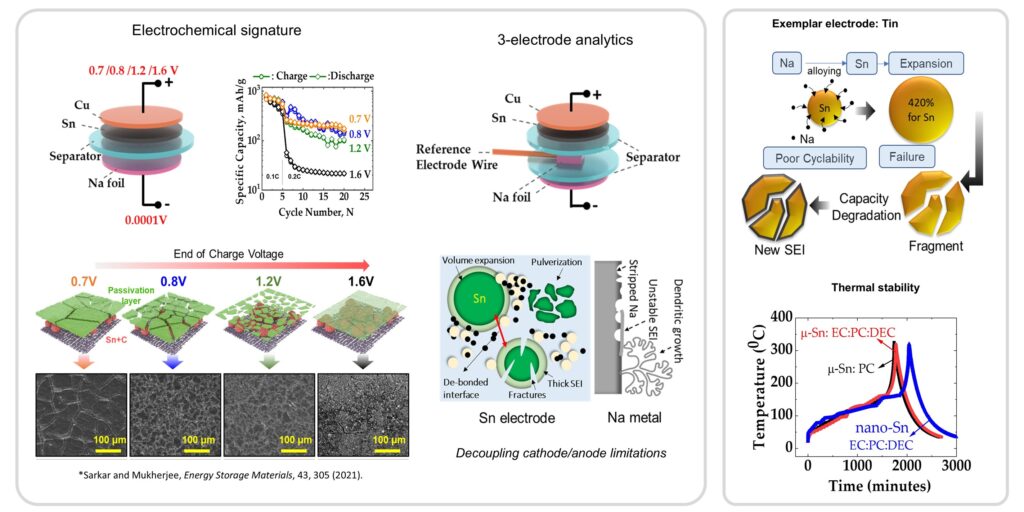
the electrode architecture will be investigated. Concerning the anode, various
candidates including carbonaceous materials, transition metal oxides, and intermetallic
and organic compounds have been studied for sodium-ion chemistry.
Based on this, the underlying reaction
mechanism is governed by insertion, conversion, or alloying. While conversion
and alloying-based anode materials can deliver higher capacities, the large
volume expansion of the host material can result in electrochemical-mechanical
instability and pose a major limitation. Based on the nature of salts, solvents,
and additives, the role of electrolyte properties including chemical,
electrochemical, and thermal stability, and ionic transport on electrochemical
performance and safety will be studied in this science vertical. For
sodium-based electrodes, the chemical composition and underlying heterogeneity
of the solid electrolyte interphase is another aspect that affects the degradation-safety
response.
Overall, the mechanistic interaction in
sodium-ion batteries including the electrode microstructure,
electrochemical-mechanical characteristics of interfaces, and
electrolyte-mediated interactions, and their implications on safety will be
analyzed.